Savings
Eligible commercially insured patients may pay as little as $0§ a month
- For covered patients
- And while insurance coverage is being established
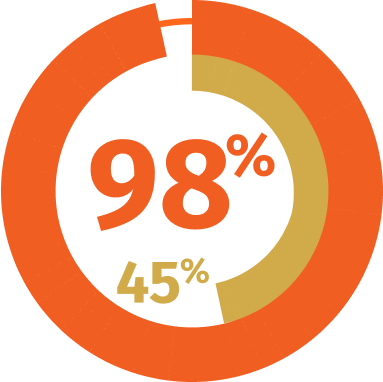
COMMERCIAL COVERAGE3*
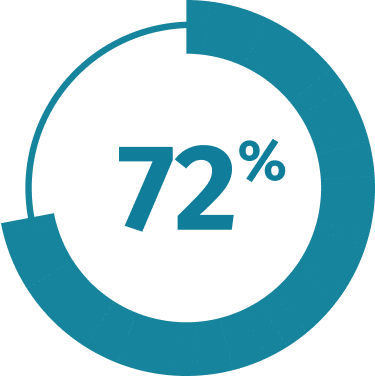
MEDICARE PART D COVERAGE3*
QULIPTA is now covered for 72% of Medicare
Part D lives!
*Managed Markets Insight & Technology, LLC™, a trademark of MMIT. Data as of August 2024 and subject to change.
Data are not a guarantee of coverage, or partial or full payment, by any payers listed. Actual benefits are determined by respective plan administrators. Insurer plans, coverage criteria, and formularies are subject to change without notice. Check each patient’s coverage with applicable insurer. AbbVie does not endorse any individual plans. Formulary coverage does not imply efficacy or safety.
†Managed Markets Insight & Technology, LLC™, a trademark of MMIT. Data as of February 2025 and subject to change.
Data are not a guarantee of coverage, or partial or full payment, by any payers listed. Actual benefits are determined by respective plan administrators. Insurer plans, coverage criteria, and formularies are subject to change without notice. Check each patient’s coverage with applicable insurer. AbbVie does not endorse any individual plans. Formulary coverage does not imply efficacy or safety.
‡Definitions:
PA: Prior Authorization; PA criteria may include diagnosis and trial and failure of one or more oral generic agents.
Preferred: QULIPTA is on a preferred tier or otherwise has preferred status on the plan's formulary.
ST: Step Therapy; ST may require trial and failure of one or more preventive treatments.
You prescribe. We provide patient support.
QULIPTA COMPLETE CAN HELP YOUR PATIENTS START AND STAY ON TRACK WITH QULIPTA IN 3 EASY STEPS

Savings
Eligible commercially insured patients may pay as little as $0§ a month

Support
Prior authorization (PA) support:
Patient support:

Not actual card.
§Eligibility: Available to patients with commercial insurance coverage for QULIPTA who meet eligibility criteria. This copay assistance program is not available to patients receiving prescription reimbursement under any federal, state, or government-funded insurance programs (for example, Medicare [including Part D], Medicare Advantage, Medigap, Medicaid, TRICARE, Department of Defense, or Veterans Affairs programs), or where prohibited by law. Offer subject to change or termination without notice. Restrictions, including monthly maximums, may apply. This is not health insurance. For full Terms and Conditions, visit QULIPTASavingsCard.com or call 1-855-QULIPTA (1-855-785-4782) for more information. To learn about AbbVie’s privacy practices and your privacy choices, visit https://abbv.ie/corpprivacy.
Online enrollment process for patients
Your eligible patients can enroll in QULIPTA COMPLETE to help them get access to a savings card and get started with QULIPTA.


Learn more about QULIPTA at upcoming speaker programs
Download QULIPTA resources.
INDICATION
QULIPTA® (atogepant) is indicated for the preventive treatment of migraine in adults.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
QULIPTA is contraindicated in patients with a history of hypersensitivity to atogepant or any of the components of QULIPTA.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions: Cases, including anaphylaxis, dyspnea, rash, pruritus, urticaria, and facial edema, have been reported with use of QULIPTA. Hypersensitivity reactions can occur days after administration. If a hypersensitivity reaction occurs, discontinue QULIPTA and institute appropriate therapy.
Hypertension (HTN): Development or worsening of pre-existing HTN has been reported following the use of CGRP antagonists, including QULIPTA. Some patients who developed new-onset HTN had risk factors. There were cases requiring initiation of HTN treatment and, in some cases, hospitalization. HTN may occur at any time but was most frequently reported within 7 days of initiation. QULIPTA was discontinued in many of the cases. Monitor patients for new-onset or worsening of pre-existing HTN, and consider whether discontinuation of QULIPTA is warranted if evaluation fails to establish an alternative etiology or blood pressure is inadequately controlled.
Raynaud’s phenomenon (RP): Development, recurrence, or worsening of pre-existing RP has been reported following the use of CGRP antagonists, including QULIPTA. In cases with small molecule CGRP antagonists, symptom onset occurred a median of 1.5 days following dosing. Many of the cases reported serious outcomes, including hospitalizations and disability, generally related to debilitating pain. In most cases, discontinuation of the CGRP antagonist resulted in resolution of symptoms. QULIPTA should be discontinued if signs or symptoms of RP develop, and patients should be evaluated by a healthcare provider if symptoms do not resolve. Patients with a history of RP should be monitored for, and informed about the possibility of, worsening or recurrence of signs and symptoms.
ADVERSE REACTIONS
The most common adverse reactions (at least 4% and greater than placebo) are nausea, constipation, and fatigue/somnolence.
Dosage form and strengths: QULIPTA is available in 10 mg, 30 mg, and 60 mg tablets.
US-QLP-250104
Please see full Prescribing Information.